Phosphorus
Phosphorus is an essential chemical element found in nature, often in the form of phosphate compounds. It is a vital component of biological molecules like DNA, RNA, and ATP (adenosine triphosphate), which play crucial roles in cellular processes and energy metabolism. In living organisms, phosphorus is integral for functions such as cell division, bone formation, and the transfer of genetic information. It is abundant in various natural sources, including foods like meat, dairy, and legumes. Phosphorus cycles through ecosystems, moving between living organisms and the environment. Its presence in water bodies can influence ecological processes, making it an important factor in environmental studies and sustainability efforts.
Facts:
Group = 15
Period = 3
Block = p
Atomic number = 15
State at 20 degrees = Solid
Electron configuration = Ne3s23p3
ChemSpider ID = 4575369
Melting point = 44.15°C
Boiling point = 280.5°C
Density = 1.823 gm cm-3
Relative atomic mass = 30.974
Key isotopes = 31p
History of Phosphorus
- Discovery by Hennig Brand (1669): German alchemist Hennig Brand is credited with the discovery of phosphorus. While attempting to create the philosopher’s stone, a substance believed to transform base metals into noble metals, Brand distilled urine and obtained a glowing material, which turned out to be phosphorus.
- Early Uses in Alchemy (17th Century): Phosphorus gained attention among alchemists and became known for its luminescent properties, making it a subject of fascination in early chemistry.
- Robert Boyle’s Studies (1680s): Irish chemist Robert Boyle conducted experiments on phosphorus, describing its properties and behavior. He also explored methods for producing it.
- Production of White Phosphorus (1769): Swedish chemist Carl Wilhelm Scheele produced white phosphorus, a more reactive and unstable form, by heating bone ash with sulfuric acid.
- Napoleonic Wars and Matches (Early 19th Century): In the early 1800s, French chemist Jean-Jacques Colin developed a process for mass-producing phosphorus for military use. Phosphorus-based munitions played a role in the Napoleonic Wars. Around the same time, English chemist John Walker invented the first friction match, which used phosphorus as a key ingredient.
- Development of Red Phosphorus (1840s): English chemist and inventor Sir H. Davy and German chemist Anton von Schrötter separately discovered a less reactive and more stable form of phosphorus, known as red phosphorus.
- Role in Agriculture (Late 19th Century): As understanding of phosphorus’s importance in plant growth grew, it became a critical component in fertilizers, significantly enhancing agricultural productivity.
- Environmental Concerns: In recent decades, the excess release of phosphorus into water bodies, often due to agricultural runoff and wastewater, has become a significant environmental issue. It can lead to problems like eutrophication, algal blooms, and oxygen depletion.
Chemical Appearance of Phosphorus
- White Phosphorus: This form is a waxy, translucent, and white-to-yellowish substance. It has a distinctive garlic-like odor and is highly reactive. White phosphorus glows faintly in the dark, a property known as phosphorescence.
- Red Phosphorus: Red phosphorus appears as a dark red, amorphous powder. It is much less reactive than white phosphorus and is not phosphorescent. Red phosphorus is stable under normal conditions.
- Black Phosphorus: Black phosphorus has a distinct layered, crystalline structure and appears as dark, metallic, or bluish-black crystals or powder. It is the most stable form of phosphorus.
Uses of Phosphorus
- Agriculture: Phosphorus is a crucial component of fertilizers. It promotes healthy plant growth, aids in root development, and supports the formation of flowers and fruits. It plays a vital role in increasing agricultural productivity.
- Food Industry: Phosphates, compounds containing phosphorus, are used as food additives in processed foods. They serve various functions, including as emulsifiers, stabilizers, and leavening agents in products like baked goods, processed meats, and dairy products.
- Detergents: Phosphates are used in detergents to enhance their cleaning effectiveness. They help remove stains, improve water softening, and prevent soil re-deposition on fabrics.
- Metallurgy: Phosphorus is used as an alloying element in steel production to enhance its properties, such as strength, hardness, and corrosion resistance.
- Fireworks: Phosphorus compounds are used in the production of fireworks to create bright and colorful displays. Different forms of phosphorus can produce a range of colors when ignited.
- Matches: Red phosphorus is a key component in safety matches, where it acts as the striking surface on the matchbox.
- Chemical Industry: Phosphorus compounds are used in the production of various chemicals, including phosphoric acid, which is a critical ingredient in the manufacture of fertilizers, detergents, and food additives.
- Electronics: Phosphorus is used in the production of semiconductors and other electronic components. It can be doped into silicon to alter its electrical properties.
- Water Treatment: Phosphates are used in water treatment processes to inhibit the formation of scale and corrosion in pipes and equipment. They also help prevent the growth of algae in water bodies.
- Healthcare and Pharmaceuticals: Phosphorus compounds are used in pharmaceuticals for various purposes, including as binding agents in tablets and as components in DNA synthesis.
- Flame Retardants: Certain phosphorus compounds are used as flame retardants in materials like textiles, plastics, and coatings to reduce their flammability.
- Lubricants and Additives: Phosphorus-containing compounds are used in lubricants and as additives in automotive and industrial applications to reduce friction and wear.
Uses of Phosphorus for Health and Body
- Bone and Teeth Health: Phosphorus is a vital component of bone mineralization, along with calcium. It contributes to the formation and maintenance of healthy bones and teeth.
- Energy Metabolism: Phosphorus is a key component of adenosine triphosphate (ATP), the primary energy currency of cells. It is involved in energy transfer and storage, supporting various metabolic processes.
- Cellular Function: Phosphorus is essential for cell growth, division, and replication. It is a fundamental element in DNA, RNA, and other nucleic acids, which carry genetic information and play critical roles in cell function.
- pH Regulation: Phosphates act as a buffer in bodily fluids, helping to regulate pH levels. This is crucial for maintaining stable and optimal conditions for cellular function.
- Nerve Function: Phosphorus is involved in nerve signaling and transmission. It helps facilitate communication between nerve cells and supports proper nervous system function.
- Muscle Function: Phosphorus is necessary for muscle contractions and relaxation. It helps regulate muscle activity, making it essential for physical movement and exercise.
- Kidney Function: Phosphorus plays a role in maintaining kidney function and filtration. Proper phosphorus levels are crucial for the kidneys to effectively remove waste products from the bloodstream.
- Cell Membrane Structure: Phospholipids, which contain phosphorus, are important components of cell membranes. They provide structure and stability to cell membranes, enabling them to function properly.
- DNA and RNA Synthesis: Phosphorus is a key component of nucleotides, which are the building blocks of DNA and RNA. It is essential for the replication and transcription of genetic material.
- Metabolism of Macronutrients: Phosphorus is involved in the metabolism of carbohydrates, fats, and proteins. It helps convert these macronutrients into usable energy for the body.
- Blood Buffering: Phosphates in the blood help maintain acid-base balance, preventing rapid changes in pH levels.
- Oxygen Transport: Phosphates are involved in the structure of red blood cells and play a role in oxygen transport in the bloodstream.
Physical Properties of Phosphorus:
- Appearance: Phosphorus exists in various allotropes. White phosphorus is a waxy, translucent, white-to-yellowish solid. Red phosphorus appears as a dark red, amorphous powder. Black phosphorus is a dark, metallic, or bluish-black crystal or powder.
- State at Room Temperature: White phosphorus is a solid at room temperature, while red and black phosphorus are also solids but have different crystalline structures.
- Melting Point: White phosphorus has a low melting point of approximately 44.1°C (111.4°F), while red phosphorus does not melt at standard atmospheric pressure.
- Boiling Point: White phosphorus boils at approximately 280.5°C (536.9°F), but it sublimes (changes directly from a solid to a gas) at lower temperatures without passing through a liquid phase. Red phosphorus does not have a distinct boiling point.
- Density: White phosphorus has a density of around 1.823 grams per cubic centimeter (g/cm³), red phosphorus has a density of about 2.2 g/cm³, and black phosphorus has a density of approximately 2.69 g/cm³.
- Solubility: Phosphorus is insoluble in water, but it can react with certain solvents and compounds under appropriate conditions.
Chemical Properties of Phosphorus:
- Reactivity: White phosphorus is highly reactive and combustible, igniting spontaneously in air. It forms compounds readily and is sensitive to heat and light. Red phosphorus is less reactive and stable under normal conditions.
- Combustibility: White phosphorus burns readily in air, producing a bright flame. It must be stored underwater or in an inert gas to prevent combustion. Red phosphorus is non-combustible.
- Oxidation States: Phosphorus can exhibit various oxidation states, including -3, +3, +4, and +5. The most common oxidation states are +3 and +5.
- Reactivity with Oxygen: Phosphorus readily reacts with oxygen to form oxides, such as phosphorus pentoxide (P4O10).
- Reactivity with Halogens: Phosphorus reacts vigorously with halogens like chlorine and bromine to form phosphorus halides.
- Reaction with Metals: Phosphorus can form alloys with certain metals, such as iron, to improve their properties.
- Reaction with Acids: Phosphorus reacts with acids to form phosphates, which are important compounds in biological systems.
- Hydrolysis: Phosphorus compounds can undergo hydrolysis reactions in the presence of water, producing phosphoric acid and related compounds.
Foods High in Phosphorus:
- Dairy Products: Milk, cheese, yogurt, and other dairy products are rich sources of phosphorus. For example, a cup of milk can provide around 247 mg of phosphorus.
- Meat and Poultry: Beef, pork, chicken, and other meats are high in phosphorus. For instance, a 3-ounce serving of cooked beef contains approximately 180-200 mg of phosphorus.
- Seafood: Fish like salmon, tuna, and trout are excellent sources of phosphorus. A 3-ounce serving of cooked salmon can supply about 280-320 mg of phosphorus.
- Nuts and Seeds: Almonds, sunflower seeds, and pumpkin seeds are among the nuts and seeds with high phosphorus content. For instance, a quarter-cup of sunflower seeds provides about 286 mg of phosphorus.
- Legumes: Lentils, chickpeas, and soybeans are good plant-based sources of phosphorus. For example, a cup of cooked lentils contains around 356 mg of phosphorus.
- Whole Grains: Whole grains like wheat bran, oats, and quinoa are relatively high in phosphorus. For instance, a cup of cooked quinoa supplies about 281 mg of phosphorus.
- Processed Foods: Some processed foods, especially those made with added phosphorus-containing additives like phosphates, can be high in phosphorus. This includes certain packaged snacks, processed meats, and pre-packaged meals.
Foods Low in Phosphorus:
- Fruits: Most fruits are naturally low in phosphorus. Examples include apples, oranges, berries, and melons. A medium-sized apple, for instance, typically contains less than 10 mg of phosphorus.
- Vegetables: Many vegetables are also naturally low in phosphorus. This includes leafy greens like spinach, kale, and lettuce. A cup of raw spinach, for example, provides only about 15 mg of phosphorus.
- Cooked Grains: While whole grains can contain moderate levels of phosphorus, when cooked, the phosphorus content is diluted. This makes options like cooked rice, pasta, and bread relatively lower in phosphorus compared to their whole grain counterparts.
- Cooked Potatoes: Potatoes, when boiled or baked, have a relatively low phosphorus content. For example, a medium-sized baked potato contains about 121 mg of phosphorus.
- Eggs: Eggs are considered low in phosphorus. One large egg provides approximately 86 mg of phosphorus.
- Dairy Alternatives: Some non-dairy milk alternatives like almond milk, rice milk, and coconut milk are typically lower in phosphorus compared to cow’s milk.
Allotropes of Phosphorus
- White Phosphorus (P4):
- Appearance: White phosphorus is a waxy, translucent, and white-to-yellowish solid.
- Structure: It consists of P4 tetrahedra, where each phosphorus atom is bonded to three other phosphorus atoms in a tetrahedral arrangement.
- Reactivity: Highly reactive and pyrophoric (ignites spontaneously in air). It’s sensitive to heat and light.
- Phosphorescence: Glows faintly in the dark due to slow oxidation.
- Uses: Used in matches, fireworks, munitions, and various chemical reactions.
- Red Phosphorus:
- Appearance: Red phosphorus is a dark red, amorphous powder.
- Structure: It has a complex polymeric structure composed of interconnected P4 tetrahedra.
- Reactivity: Less reactive and stable under normal conditions. It is not pyrophoric.
- Uses: Used in safety matches, flame retardants, and certain chemical reactions.
- Black Phosphorus:
- Appearance: Black phosphorus appears as dark, metallic, or bluish-black crystals or powder.
- Structure: It has a layered, crystalline structure similar to graphite, with puckered layers of P4 units.
- Reactivity: More stable than white phosphorus but less reactive than red phosphorus.
- Uses: Used in electronic devices, semiconductors, and as a lubricant.
Compounds of Phosphorus
- Phosphates (PO4^3-): Phosphates are salts or esters of phosphoric acid (H3PO4). They are fundamental in biological systems, serving as essential components of DNA, RNA, ATP (adenosine triphosphate), and various other biomolecules. Examples include calcium phosphate (found in bones and teeth) and sodium phosphate (used in food additives).
- Phosphoric Acid (H3PO4): A key inorganic acid, phosphoric acid is a tribasic acid, meaning it can donate up to three protons (H+) in solution. It’s widely used in industries for manufacturing fertilizers, detergents, and food additives.
- Phosphine (PH3): Phosphine is a colorless, flammable gas composed of one phosphorus atom bonded to three hydrogen atoms. It is used in various industrial applications, including as a fumigant for stored grain.
- Organophosphates: These are compounds containing carbon-phosphorus bonds. They are used in pesticides, herbicides, and nerve agents (e.g., sarin gas). Examples include insecticides like malathion and herbicides like glyphosate.
- Phosphorus Oxyacids: These are acids containing phosphorus, oxygen, and hydrogen. Examples include:
- Hypophosphorous Acid (H3PO2): Used as a reducing agent in chemical reactions.
- Phosphorous Acid (H3PO3): Used as a fungicide and in the manufacture of flame-retardant materials.
- Hypophosphoric Acid (H4P2O6): An unstable compound with two P-O-P bonds.
- Phosphorothioates: These are organophosphates where one or more oxygen atoms are replaced by sulfur atoms. They are used in certain pesticides and DNA-modifying agents.
- Phosphides: Compounds formed between phosphorus and metals, such as aluminum phosphide (used as a fumigant) and calcium phosphide (used in fireworks).
- Phosphonium Compounds: These are salts containing a positively charged phosphorus atom bonded to four organic groups. They have various applications in organic synthesis.
- Phosphazenes: These are compounds with a central phosphorus atom bonded to alternating nitrogen atoms. They have applications in materials science and catalysis.
- Phosphonyl Compounds: These contain a phosphorus atom bonded to an oxygen atom and an organic group. They find applications in flame retardants and pharmaceuticals.
Phosphate
Phosphorus is an essential chemical element found in nature, often in the form of phosphate compounds. It is a vital component of biological molecules like DNA, RNA, and ATP (adenosine triphosphate), which play crucial roles in cellular processes and energy metabolism. In living organisms, phosphorus is integral for functions such as cell division, bone formation, and the transfer of genetic information. It is abundant in various natural sources, including foods like meat, dairy, and legumes. Phosphorus cycles through ecosystems, moving between living organisms and the environment. Its presence in water bodies can influence ecological processes, making it an important factor in environmental studies and sustainability efforts.
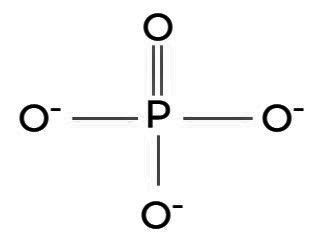
Structure of Phosphate
Phosphates have a tetrahedral molecular structure. In a typical phosphate ion (PO4^3-), there is one central phosphorus atom bonded to four oxygen atoms. Each oxygen atom forms a single covalent bond with the phosphorus atom. The phosphate ion carries a negative charge, as it has one more oxygen atom than it has hydrogen atoms to balance the charge.
This structure gives phosphates distinctive chemical properties, making them essential components in various biological processes, including DNA and RNA formation, energy metabolism, and the buffering of bodily fluids. The arrangement of atoms in a phosphate ion allows it to participate in crucial reactions and interactions within living organisms and inorganic chemical systems alike.
Physical Properties of Phosphate:
- State at Room Temperature: Phosphates can exist in various states, depending on their specific chemical composition. Common forms include solid minerals, powders, and crystalline compounds.
- Solubility: Many phosphates are soluble in water, while others may be insoluble or have limited solubility. For example, sodium phosphate is highly soluble, whereas calcium phosphate is less soluble.
- Melting and Boiling Points: The melting and boiling points of phosphates vary widely depending on their specific chemical structure and the elements they are bonded to.
- Density: The density of phosphates varies depending on their specific form and composition.
Chemical Properties of Phosphate:
- Reactivity: Phosphates are reactive chemical species, especially in aqueous solutions. They can undergo various chemical reactions, including precipitation, dissociation, and complex formation.
- Acidity or Basicity: Depending on the specific phosphate compound, it can act as an acid or a base in chemical reactions. For instance, phosphoric acid (H3PO4) is a triprotic acid, meaning it can donate up to three protons (H+) in solution.
- Complex Formation: Phosphates have the ability to form complexes with metal ions, which is important in many biological processes and in environmental chemistry.
- Buffering Capacity: Phosphates are known for their buffering capacity, meaning they can resist changes in pH when an acid or base is added to a solution. This property is crucial in biological systems.
- Redox Reactions: Some phosphates, particularly those containing multiple phosphorus atoms, can participate in redox reactions, where electrons are transferred between molecules.
- Biological Significance: Phosphates play a critical role in biological systems. They are integral components of DNA, RNA, ATP (adenosine triphosphate), and other important biomolecules.
- Precipitation Reactions: Phosphates can form insoluble salts with certain metal ions, which leads to the formation of minerals in natural environments.
- Hydrolysis: Some phosphate compounds can undergo hydrolysis reactions in the presence of water, breaking down into their constituent ions.
Uses of Phosphate
- Agriculture:
- Fertilizers: Phosphates are a crucial component of fertilizers, providing essential nutrients like phosphorus to promote plant growth and development.
- Food Industry:
- Food Additives: Phosphates are used as food additives to enhance texture, flavor, and shelf life. They are commonly found in processed meats, baked goods, and dairy products.
- Water Treatment:
- Phosphate-Based Water Softeners: They are used to remove calcium and magnesium ions from hard water, preventing scale buildup in pipes and appliances.
- Detergents:
- Builders: Phosphates act as water softeners and help remove mineral deposits in laundry detergents, improving their cleaning efficiency.
- Metallurgy:
- Metal Cleaning: Phosphates are used in metal cleaning and surface treatment processes to prepare surfaces for painting or coating.
- Medical and Pharmaceutical Industry:
- Buffering Agent: Phosphates are used as buffering agents in pharmaceutical formulations to maintain a stable pH level.
- Fireworks and Pyrotechnics:
- White Phosphorus: White phosphorus is used in the manufacture of fireworks due to its pyrotechnic properties.
- Industrial Processes:
- Corrosion Inhibitors: Phosphates are used in cooling water treatment to prevent corrosion in industrial equipment.
- Cleaning Products:
- Household Cleaners: Phosphates can be found in some household cleaning products, where they help remove stains and mineral deposits.
- Environmental Applications:
- Phosphate Removal: Phosphate removal is crucial in wastewater treatment to prevent eutrophication (excessive plant growth) in natural water bodies.
- Energy Production:
- Batteries: Certain types of batteries, such as nickel-cadmium batteries, use phosphates as electrolytes.
- Construction Industry:
- Concrete Additives: Phosphates can be used as additives in concrete to improve its workability and strength.
- Biological Systems:
- Cellular Energy Transfer: Phosphates are a fundamental component of ATP (adenosine triphosphate), the primary molecule for energy transfer in cells.
- DNA and RNA:
- Genetic Material: Phosphates form the backbone of DNA and RNA molecules, providing structural stability.
- Bone and Teeth Health:
- Calcium Phosphate: It is a major component of bones and teeth, providing strength and structure.
Hazards of Phosphate
- Water Pollution:
- Eutrophication: Excessive phosphate runoff from agricultural fields and urban areas can lead to eutrophication, where an overabundance of nutrients in water bodies promotes the rapid growth of algae and aquatic plants. This can deplete oxygen levels, harm aquatic life, and disrupt the balance of the ecosystem.
- Groundwater Contamination:
- Leaching: Phosphate fertilizers can leach into the groundwater, potentially contaminating drinking water sources. Elevated phosphate levels in drinking water can be harmful to human health, particularly for individuals with kidney disorders.
- Algal Blooms:
- Harmful Algal Blooms (HABs): Excessive phosphates in water bodies can lead to the proliferation of harmful algal species. Some of these species produce toxins that can be harmful to aquatic life and pose risks to human health if consumed.
- Aquatic Habitat Degradation:
- Loss of Biodiversity: Eutrophication and algal blooms can lead to oxygen depletion in water bodies, causing stress to fish and other aquatic organisms. This can lead to fish kills and a decline in overall biodiversity.
- Soil Degradation:
- pH Imbalance: Overuse of phosphate-containing fertilizers can lead to soil pH imbalances, affecting the availability of other essential nutrients for plant growth. This can lead to decreased soil fertility and productivity.
- Health Risks:
- Excessive Dietary Intake: Consuming food products with high levels of added phosphates (e.g., processed foods) can lead to an excessive intake of phosphates, potentially causing health issues, particularly for individuals with kidney problems.
- Corrosion and Environmental Damage:
- Industrial Processes: Improper handling and disposal of phosphate-containing chemicals in industrial settings can lead to corrosion of equipment and potential environmental contamination.
- Bioaccumulation and Biomagnification:
- Toxic Effects: In aquatic environments, some forms of phosphates can be absorbed by aquatic organisms. Through the process of bioaccumulation and biomagnification, higher trophic level organisms can accumulate harmful levels of phosphates, leading to potential toxic effects.
- Altered Soil Microbial Communities:
- Microbial Disruption: Excessive phosphate application can alter the composition of soil microbial communities, potentially disrupting nutrient cycling processes and overall soil health.
- Regulatory Concerns:
- Environmental Regulations: In many regions, there are strict regulations governing the use and disposal of phosphates to mitigate potential environmental hazards.
Important Differences between Phosphorus and Phosphate
| Basis of Comparison | Phosphorus | Phosphate |
| Chemical Composition | An element, represented by the symbol P. | A chemical compound containing phosphorus. |
| Charge | Neutral | Usually carries a negative charge (-3). |
| State at Room Temp. | Can exist as various allotropes, e.g., white phosphorus, red phosphorus. | Typically exists as a solid or in dissolved form in water. |
| Occurrence in Nature | Found in various minerals, rocks, and organic matter. | Abundantly present in minerals, especially as orthophosphates. |
| Role in Biological Systems | Essential element for all living organisms, key component of biomolecules like DNA, RNA, and ATP. | Vital for energy transfer in cells (ATP), structural support (bone mineralization), and metabolic processes. |
| Forms | Can exist in multiple allotropes, including white, red, and black phosphorus. | Exists in various forms, such as orthophosphates, pyrophosphates, and polyphosphates. |
| Reactivity | Highly reactive, especially in contact with oxygen and water. | Reactive, especially in aqueous solutions, participating in various chemical reactions. |
| Industrial Applications | Used in various industries, including metallurgy, electronics, and chemical synthesis. | Widely used in agriculture (fertilizers), food industry (additives), water treatment, and detergents. |
| Environmental Impact | Can be a pollutant if released in excessive amounts, leading to ecological imbalances. | Excessive phosphate runoff can lead to eutrophication, causing harm to aquatic ecosystems. |
| Health Implications | Toxic in certain forms (e.g., white phosphorus), potentially harmful if ingested or inhaled. | Generally safe when consumed in natural forms (e.g., inorganic phosphate salts), but excessive intake can lead to health issues. |
| Common Compounds | Elemental phosphorus, phosphoric acid (H3PO4), organophosphates. | Orthophosphates (e.g., HPO4^2-), pyrophosphates, polyphosphates, phosphoric acid, etc. |
| Industrial Uses | Used in the production of matches, fireworks, electronic devices, and as a reducing agent in chemical processes. | Used in fertilizers, food additives, water treatment, detergents, and various industrial applications. |
| Environmental Regulations | Strict regulations govern the handling and disposal of elemental phosphorus due to its potential hazards. | Regulations exist for the use and discharge of phosphates, especially in industries with potential environmental impact. |
| Allotropes | Can exist in multiple allotropes, each with distinct properties. | Phosphate ions do not have allotropes, as they are chemical ions composed of phosphorus and oxygen atoms. |
Similarities between Phosphorus and Phosphate
- Elemental Composition: Both phosphorus and phosphate contain the element phosphorus (P) as a central component.
- Biological Importance: Both are crucial for living organisms. Phosphorus is an essential element for all forms of life, and phosphate ions play vital roles in biological processes.
- Role in Energy Transfer: Both are involved in energy transfer processes in living organisms. Phosphorus is a key component of ATP (adenosine triphosphate), the primary molecule for energy storage and transfer.
- Found in Nature: Both occur naturally in various forms. Phosphorus is found in minerals, rocks, and organic matter, while phosphates are abundant in minerals and play a crucial role in soil and water chemistry.
- Forms in Chemical Reactions: Phosphorus and phosphate can participate in various chemical reactions, including redox reactions, acid-base reactions, and complex formation.
- Environmental Impact: Both can have environmental implications. Excessive amounts of phosphorus and phosphate runoff can lead to ecological imbalances, such as eutrophication in water bodies.
- Industrial Applications: Both are used in various industrial processes. Phosphorus is utilized in industries like metallurgy, electronics, and chemical synthesis. Phosphates find applications in agriculture, food additives, water treatment, and detergents.
- Health Considerations: Both have health implications. Elemental phosphorus can be toxic in certain forms, while excessive intake of phosphate compounds can lead to health issues, especially for individuals with kidney problems.
- Compounds and Forms: Both have various forms and compounds. Elemental phosphorus can exist in different allotropes, while phosphate ions come in various forms, including orthophosphates, pyrophosphates, and polyphosphates.
Advisory Note: Article shared based on knowledge available on internet and for the Knowledge purpose only. Please contact Professional/Advisor/Doctor for treatment/Consultation.
Articles on intactone.com are general information, and are not intended to substitute for Professional Advice. The information is “AS IS”, “WITH ALL FAULTS”. User assumes all risk of Use, Damage, or Injury. You agree that we have no liability for any damages.